Mycorrhization leads to nutrient and information flow, often in both directions. The plant root supplies sugars to the fungus, while the fungus induces Jasmonic Acid biosynthetic enzymes in the plant, leading to an increase in jasmonate levels that enhance the accumulation of soluble sugars in plant root and the production of plant root defense compounds.
From a research article, the presence of mycorrhizal fungus, Glomus mosseae and nitrogen fixing Bacillus subtilis on the roots influenced the levels of plant biomass growth, and the yield of an important medicinally active phytochemical, artemisinin, from Artemisia annua L and used as an anti-malarial treatment.
Gabriele et al. (2016) investigated the effect of mycorrhizal soil inoculation of various Sangiovese wine grapes and found the presence of the fungus increased levels of 14 polyphenols compared to un-inoculated plants. Here the presence of symbiotic relations in the soil altered the phytochemical makeup of fruit.
So how are the plant roots attracting mycorrhizal symbionts? Plant produced flavanoid compounds accumulate at root tips/cap and make up a large portion of root exudate (the portion of the root sap excreted to the external environment). These phytochemicals are easily modified and their biosynthesis is triggered by transcription factors, which suggests a role as elicited signal compounds – compounds that are made specifically in response to conversation from rhizosphere fungi and bacteria. Interestingly, their presence in the rhizosphere soil triggers mycorrhizal fungi to explore their surroundings (Hassan and Mathesius, 2012), perhaps increasing the likely hood of contact with plant roots.
Given the high price of American wild grown ginseng, the ecological influence on ginsenoside formation, and ultimately, the therapeutic value, points to optimizing the rhizosphere cross talk by way of forest farming.
The highest ginsenoside content occurs (from highest to lowest) in the root hairs > lateral roots > cortex > interior taproot (Li and Wardle, 2002), exactly where we should expect a chemical conversation to occur.
Within this class of compounds we designate as ginsenosides, two molecular forms are dominant, protopanaxadiols and protopanaxatriols. Data from two different papers (Zhu et al., 2004: Wang et al., 2010) compared levels of diols and triols in different species and sources of ginseng. American ginseng (Panax quinquefolia) had higher levels of the triols (especially Rg1) compare to Chinese ginseng (P. ginseng), which had higher levels of diols (especially Rb1 Rd).
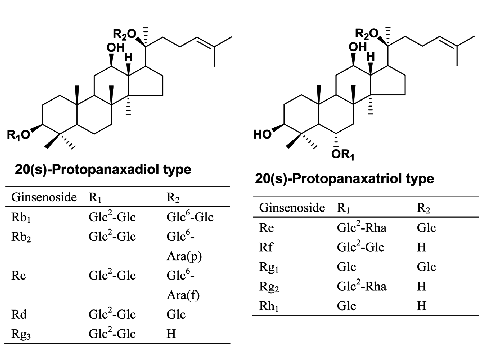
Comparing wild grown versus cultivated plants within each species, a similar pattern emerged, with wild plants showing a higher concentration of triols (especially Rg1 Re), while cultivated plants had higher concentration of diols (especially Rb1 Rb2).
James, et al. (2013) investigated levels of diols and triols in wild sourced P. quinquefolia leaf and root in a North Carolina collection, finding that there was no relationship between age and ginsenoside content. However total ginsensosides were higher in the leaf, as was Rb2 and Rd (diols), In the root tissue, Rb1(diol) and Rg1 (triol) was found to be higher.
This has implications for how we “farm” medicine and speaks to a long held tenet; complex interactions in native ecologies, including the soil, produce medicinal plant crops that are more biologically active. Farm versus wild grown ginseng is only one example. What’s been your experience as a imbiber, herbalist, researcher, plant grower or manufacturer?

 Understanding dormancy requirements for woodland, medicinal plant species is a requirement for discovering how they initiate their relationship with soil fungi. Sanders & McGraw (2002) noted that despite wide geographic distribution, seedling establishment is a constraint in wild goldenseal (Hydrastis canadensis, L.) populations. Richo Cech, of
Understanding dormancy requirements for woodland, medicinal plant species is a requirement for discovering how they initiate their relationship with soil fungi. Sanders & McGraw (2002) noted that despite wide geographic distribution, seedling establishment is a constraint in wild goldenseal (Hydrastis canadensis, L.) populations. Richo Cech, of