I was lucky enough to grow up all over the world – Thailand, Hong Kong, Germany, Romania and Greece. Some of my fondest memories are around food. My mom was a fabulous cook, which was a vital part of my father’s work as a cultural affairs officer in the US Foreign Service.

One of my earliest memories was of making a landing in Anchorage Alaska on a return trip from Bangkok to my parents’ home town of Biloxi, Mississippi. I remember the large stuffed polar bear in the lobby, and more importantly, my first taste of vanilla ice cream. Having come from the heat of Bangkok, with my daily food rich in hot peppers and ginger, this cooling fragrant treat was nectar from the gods.
When I started to learn to cook standing over my mom’s pot of gumbo she stressed the importance of simmer with the last ingredients – two bay leaves. They looked so simple – small dried plant material – but an hour later, the aroma of my Nannie’s gumbo was bigger than just the file and seafood, with the bay leaves providing a vibrant top note. Years later, deep into a career researching active molecules in medicinal plants, I realized that the food culture I grew up in was full of medicinal plants in the spices added to our evening meals.

Spices both sense of taste and smell. Our sense of smell, in particular, is influenced by the characteristics of the molecules responsible for odor. How quickly do they volatilize (how easily molecules vaporize)? How soluble are they in water or oil? How acidic is the food (a tomato based food or something with lemon)? Even our genes can influence the sense of smell.
A molecule that we can sense through smell is called a fragrance or odorant. Those molecules need to reach the olfactory system in the upper part of our nose. To do so, generally the molecular weight of these molecules needs to be equal to or less than 300 g/mol (a scientific designation for the mass of a substance divided by the amount of the substance). Ultimately, our sense of smell comes down to a pattern of activity of neurons in the brain responding to the stimulus of odor molecules binding to receptors (Malnic et al., 1999).
We have receptors (a protein in a cell membrane that responds to molecule attaching to it) in our nasal passages that are triggered by these molecules. Each olfactory receptor recognizes more than one odorant, and each odorant can be detected by several different olfactory receptors. This reflects a combinatorial process common to biological systems. Several factors influence the resulting signal sent from the receptor site.
We know that the shape of a molecule is important (Saberi and Seyed-Allaei, 2016), and that as the molecule binds (or sticks to) a receptor, the receptor changes shape, which leads to neural signals reaching the brain. Several theories exist to help us understand how to map the process of detecting odors, based largely on chemical qualities of the odorants.
Mori et al. (1994) suggested that the odor signal are more complex than a single receptor binding event, rather the signal is a product of a series of receptor excitations from numerous receptor sub sites (odotypes). This became known as the Odotope Theory. This theory also explains odor-less molecules as the presence sub sites more numerous than the limit of potential binding sites.
A second important theory, Dyson (1938) originated the Vibrational Theory that Wright (1982) later refined. This theory posits that olfactory receptors might really sense vibrational energy on a quantum level rather than structural shapes of the molecule when detecting odors. Quantum mechanics describes nature at the smallest scales of energy levels of atoms or substances.
Combining the two theories, Turin and Yoshi suggest that the tightness of receptor binding, based on both the physical and charge shape (polarity) of an odorant molecule may influence the intensity of the odor, while the character of the odor is effected by vibrational characteristics of the molecule.
To help visualize the theories, we can take a series of molecules found in medicinal plants that have a common base structure, and explore how their odor characteristics may be influenced by chemical features such as solubility, volatility, molecular shape and size (see Table 1).
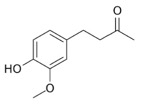
Starting with the basic structure, vanillin give vanilla its signature sweet, perfumed, woody aroma. The molecular weight is relatively low, and it volatilizes easily, filling a room with the odor when cooked. The oxygen R-groups (groups that “hang” from the ring) on the benzene ring of vanillin make it highly solubility in water.
Built from the same basic structure, eugenol has a short hydro-carbon tail that gives it a stronger odor than vanillin, the familiar aroma found in bay leaf or allspice. This hydro-carbon tail also makes it more fat soluble and may influence the intensity of receptor binding. The odor threshold of eugenol is also lower than that of vanillin. The less polar binding site on the molecule may influence the strength of binding in this example, and explain why the vanillin odor is not as persistent as that of bay leaf or allspice.
Table 1: Vanillin Based Molecular Structures with Odor Thresholds
 |
 |
| Vanillin
(Odor Threshold: 20-200ppb)* |
Eugenol
(Odor Threshold: 6-30ppb) |
 |
 |
| Zingerone
(Odor Threshold: na) |
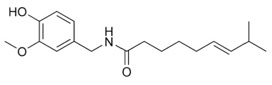
Capsaicin
(Odor Threshold: na) |
*http://www.leffingwell.com/odorthre.htm

Zingerone has an even longer hydro-carbon tail connected to the basic vanillin shape, making it insoluble in water. Found in ginger and mustard oil, it gives a rich, sweet, warm and woody fragrance. However, the presence of the carbonyl group (C=O) in the tail means that the zingerone molecules tend to attract each other, limiting how easily it volatilizes. Thus, ginger is less likely to fill a room as quickly with its aroma than vanilla.
Even though Capsaicin also has a long, hyrdo-carbon rich tail, the polar amide group (-NHCO-) makes it slightly more soluble in water than zingerone. The size of the tail also limits the molecule’s volatility. Capsaicin has no odor, and given the numerous sites along that long tail with the potential of binding to a receptor sub sites, this may be an example of a molecule with too many binding sites, as the Odotope theory suggests.

So the next time in you are in the kitchen and the aromas are filling your senses, see if you can think about the shapes and characteristics of the molecules influencing that wonderful moment.
References:
- Malnic B, Hirono J, Sato T, Buck LB. (1999) Combinatorial receptor codes for odors. 96(5), 713.
- Saberi M, Seyed-Allaei H (April 2016). “Odorant receptors of Drosophila are sensitive to the molecular volume of odorants”. Scientific Reports. 6: 25103. doi:1038/srep25103.
- Mori, K. and Shepherd, GM. (1994). Emerging principles of molecular signal processing by mitral/tufted cells in the olfactory bulb. Semin Cell Biol 5-1:65-74.
- Dyson, G.M. The scientific basis of odour . Chem Ind 57: 647-651.
- Wright, R.H. (1982) The sense of smell CRCpress, Boca Raton, Florida, USA.
- Turin, L, and Yoshi, F. (2003) Structure-Odor Relations: a modern perspective in Handbook of Olfaction and Gustation Second Edition. Doty RL, Ed. New York Marcel Dekker.
Pingback: The Real Gumbo Recipe – Bhodi Tims