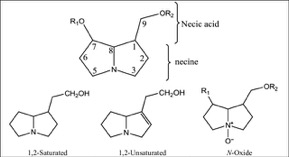
The theory of evolutionary radiation of flowering plants has been linked to interaction with pollinators and herbivores (Ehrlich and Raven, 1964: Herrera et al., 2002). One class of phytochemicals that researchers investigated to understand the mechanisms for a co-evolutionary impact is pyrrolizidine alkaloids (PAs). Much of the past research has been on the toxicity of these compounds in various structural iterations. When they contain 1,2 double-bond (unsaturated) in their base, necine moiety (Figure 1), PAs can be activated to become hepatotoxic, carcinogenic, genotoxic and teratogenic to humans (IPCS 1988).

This toxicity impacted the dietary supplement market in 2001, when the USA FDA required withdrawal of PA-containing Comfrey (Symphytum officinalis) preparations from the market. Nevertheless, other organisms have benefited from ingesting the compounds.
Biological data revealed how insects interactions with PA containing plants can be protective to the insect (Berenbaum and Aangerl, 1993; Nelson and Kursar, 1999). More recently, Liu, Vrieling and Klinkhamer (2018) explored these synergistic effects with herbivores. Their study evaluated the anti-herbivory effect of PAs on the study insects, Western Flower Trips (WFT). They found that plant secondary metabolite fractions underestimates the effect on herbivory. All fractions contained N-oxides and free bases. Most of the anti-herbivory effect was restored when fractions were recombined.
The insects were more susceptible to the more polar fraction of butanol than the chloroform fraction. Both N-oxides and free bases were present in higher concentration in the butanol as opposed to the chloroform, but total PA amounts were 3x
greater in later. They also noted that when combined with chlorogenic acid (CGA), the N-oxide was more active than when combined with free base, which is the opposite of what was expect based on toxicity profiles.
One of the more important findings from their study was that aside from the N-oxide and freebase forms of the PA, other metabolites can act as antagonists or synergists between CGA and the PA retrorsine. The complexity of the phytochemical background altered the interactions between plant metabolites and their potential bioactivity. What we don’t know is if the use of laboratory spiking studies will work in the field, and will it have population level effects necessary for long term alterations to behavior?
Another piece of interesting research focused on where these compounds get sequestered in the plant. Stegemann et al. (2018) investigated the accumulation and role of PAs’ in comfrey (Symphytum officinale) flowers and fruits. It was originally believed that PA synthesis occurred in the plant roots, which was then distributed to the rest of the structure. They reported secondary sites of synthesis in young leaves subtending developing inflorescences, with transport from leaf to flower to protect reproductive structures.
The authors found variability in accumulation patterns in different tissue, strongly suggesting that the synthesis is developmentally driven. Generally, the level of N-oxides to tertiary PAs was ~95% in all tissue , and the fresh weight PA concentration (ppm) present in different plant parts are listed as follows (from least to most):
- sepals (tr)
- petals (6)
- pollen (14)
- ovaries (98)
- complete flower (141)
- fruits (183)
The patterns of the alkaloids themselves were different in various tissue. Application of methyl jasmonate didn’t appear to alter the levels of expression, thus they appear not to be constitutively produced. The highest levels appeared at peak inflorescence, dropping as the flowers withered. And additional PAs are stored in young leaves and reproductive structures.
In table below, even though only trace amounts of all PAs are located in petals and pollen, almost all is the PA is myosorpine. It’s not clear why that pattern of individual PAs is unique to specific tissue. The presence of specific PAs in different tissues may be “leftover” from the original metabolic pathway, with the plant shuttling those particular metabolites to specific tissue.
We can compare these unique distribution and accumulation patterns to plants that normally don’t produce PAs. Now et al. (2016) tested horizontal transfer of natural products from leachate of rotting plants containing PAs into plants that do not produce those compounds. The accumulation of PA’s occurred in the leaves of guest plant, not flowers, suggesting xylem transfer driven via transpiration. Accumulation in flowers via transport (as opposed to being a production site) is typically source-sink-translocation via phloem. The researchers found that the PAs accumulated as salt like N-oxides. They had expected the presence of free base PAs since they are able to cross biomembranes via simple diffusion. Either the guest plant has a transporter able to translocate N-oxides, or the free base PAs are taken up and oxidized to N-oxide. It made me wonder if this root uptake of exogenous PA’s might happen in all plant species, or was it limited specific plant species, genus or families?
Finally, Wink (2019) discussed how PAs are often stored by specialized insects and that their chemical ecology is intricate. As an example Wink noted how they function as nuptial gift for defense of eggs in the caterpillar larvae of Creatonotus spp. after metamorphosis into adult insects. The presence of the PAs secreted on to the eggs protects them from potential predators. Because of that benefit, it appears that increased PA ingestion by male caterpillars enhances their attractiveness.
This last puzzle in the world of PA chemical ecology had me wondering what examples might exist in the human world that mimic this type of behavior. Although beer consumption has long been thought to enhance the appearance of human male virility, my own experience has disproved that particular absurdity. Do you have any examples?
References:
- Berenbaum MR, Zangerl AR (1993) Furanocoumarin metabolism in Papilio polyxenes: biochemistry, genetic variability, and ecological significance. Oecologia 95:370–37. https://doi.org/10.1007/BF00320991.
- & (1964) Butterflies and plants: a study in coevolution. Evolution 18: 586– 608. DOI: 10.2307/2406212
- El Sahzly, A. and Wink, M. (2014) Diversity of Pyrrolizidine Alkaloids in the Boraginaceae Structures, Distribution, and Biological Properties. Diversity 6(2):188 – 282. https://doi: 10.3390/d6020188.
- International Programme on Chemical Safety (IPCS) (1988) Pyrrolizidine alkaloids. Environmental health criteria 80. WHO, Geneva.
- Herrera, C.M., Medrano, M., Rey, P.J., Sánchez-Lafuente, A.M., García, M.B., Guitián, J., and Manzaneda, A.J. (2002) Interaction of pollinators and herbivores on plant fitness suggests a pathway for correlated evolution of mutualism- and antagonism-related traits. PNAS. 99 (26): 16823-16828. DOI: 10.1073/pnas.252362799.
- Liu, X., Vrieling, K., and Klinkhamer, PGL. (2018) Phytochemical background medicates effects of pyrrolizidine alkaloid in Western Flower Trips. J. Chem. Eco. 45(2): 116–127.
- https://doi.org/10.1007/s10886-018-1009-2.
- Nelson AC, Kursar TA (1999) Interactions among plant defense compounds: a method for analysis. Chemoecology 9:81–92. https://doi.org/10.1007/s000490050037.
- Now, M., Wittke, C., Lederer, I., Klier, B., Kleinwächter, M., and Selma, D. (2016) Interspecific transfer of pyrrolizidine alkaloids: An unconsidered source of contaminations of phytopharmaceuticals and plant derived commodities. Food Chem. 213: 163–168. https://doi.org/10.1016/j.foodchem.2016.06.069.
- Sahzly, A. and Wink, M. (2014) Diversity of Pyrrolizidine Alkaloids in the Boraginaceae Structures, Distribution, and Biological Properties. Diversity 6(2):188 – 282. https://doi: 10.3390/d6020188.
- Stegemann T, Kruse LH, Brütt M, and Ober D. (2018) Specific Distribution of Pyrrolizidine Alkaloids in Floral Parts of Comfrey (Symphytum officinale) and its Implications for Flower Ecology. J Chem Ecol. doi: https://10.1007/s10886-018-0990-9.
- Wink, M. (2019) Quinolizidine and Pyrrolizidine Alkaloid Chemical Ecology – a Mini-Review on Their Similarities and Differences. J Chem Ecol. 45(2):109-115. https://doi.org/10.1007/s10886-018-1005-6.